Qiang Chen, Marshall Bergen and James F. White, Jr.
Department of Plant Biology and Pathology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901
About The Author
Qiang Chen is a doctoral student in Department of Plant Biology at Rutgers University. His research focuses on the bacterial
endophytes from turf grasses. He intends to graduate in 2017 and hopes to work as a researcher studying bacterial endophytes and their functions, either in the public or private sectors.
Introduction
The existence of non-pathogenic microbes in plants has long been known. These microbes are referred to as endophytes. Endophytes are microbes that enter into the tissues of plants without causing disease. Both fungal and bacterial endophytes are common in plants. How most endophytes interact with plant hosts is still unknown, but several studies demonstrate that endophytes may benefit plants by increasing their competitive abilities and overall tolerance to stress.
In turf grass, fungal Epichloë endophytes have been well known to enhance plant growth and resistance to biotic and abiotic stresses. However, the presence and effects of bacterial endophytes in turf grass are less well known. In our study, we examined seed-transmitted bacterial endophytes in Chewing’s fescue, creeping red fescue, hard fescue, Kentucky bluegrass, perennial ryegrass, sheep fescue, slender creeping red fescue and tall fescue. Also, we evaluated the effects of endophytic bacteria on the plants.
Materials And Methods
Bacterial Isolation and Identification
Seeds of eight turfgrass species were obtained from breeding program selections available at the Rutgers Research Farm in Adelphia, N.J. To remove surface bacteria, grass seeds were surface disinfected in 4 percent sodium hypochlorite solution for 30 minutes and rinsed three times with water for 30 seconds. The surface disinfected seeds were put on Potato Dextrose Agar (PDA; Difco Laboratories, Detroit, Mich.) and 10 percent Tripticase Soy Agar (TSA; Difco Laboratories, Detroit, Mich.) for isolation of bacteria.
For bacterial identification, about 1,450 base pairs of the 16S rDNA region were amplified with primers 16S-27F (5’- AGAGTTTGATCMTGGCTCAG-3’) and 16S-1492R (5’- AAGGAGGTGWTCCARCC-3’) (M=A/C, W=A/T, R=A/G). Polymerase chain reaction (PCR) amplification was conducted with the program of denaturing at 94oC for 60 seconds, annealing at 46oC for 30 seconds and extension at 72oC for 90 seconds, 36 cycles. The PCR products were checked using agarose gel electrophoresis (1.2 percent) and then sent for sequencing to Genewiz, Inc (Plainfield, N.J.). The sequencing results were compared on NCBI for identification.
Seed Germination Test
Seeds of Kentucky bluegrass (P. pratensis) were disinfected in 4 percent sodium hypochlorite solution for 30 minutes and rinsed three times with sterile water for 30 seconds. Bacterial isolates Bacillus amyloliquefaciens and Bacillus pumilus from sheeps fescue and two Pantoea agglomerans isolates from tall fescue (F. arundinacea) and teosinte (Zea mays Mexicana) were cultured in Potato Dextrose Brouth. After 24 hours, bacteria were concentrated by centrifugation and then diluted in water to OD600=0.8. Surface disinfected seeds of Kentucky bluegrass were inoculated with bacterial solutions by soaking with agitation for five minutes. Then seeds were placed on 1.5 percent water agar and 1.5 percent agar with 100mM NaCl. After a 7, 10, 14 and 17 day incubation period on the media, the rates of germination were calculated for each treatment.
Salt Stress Tolerance Test
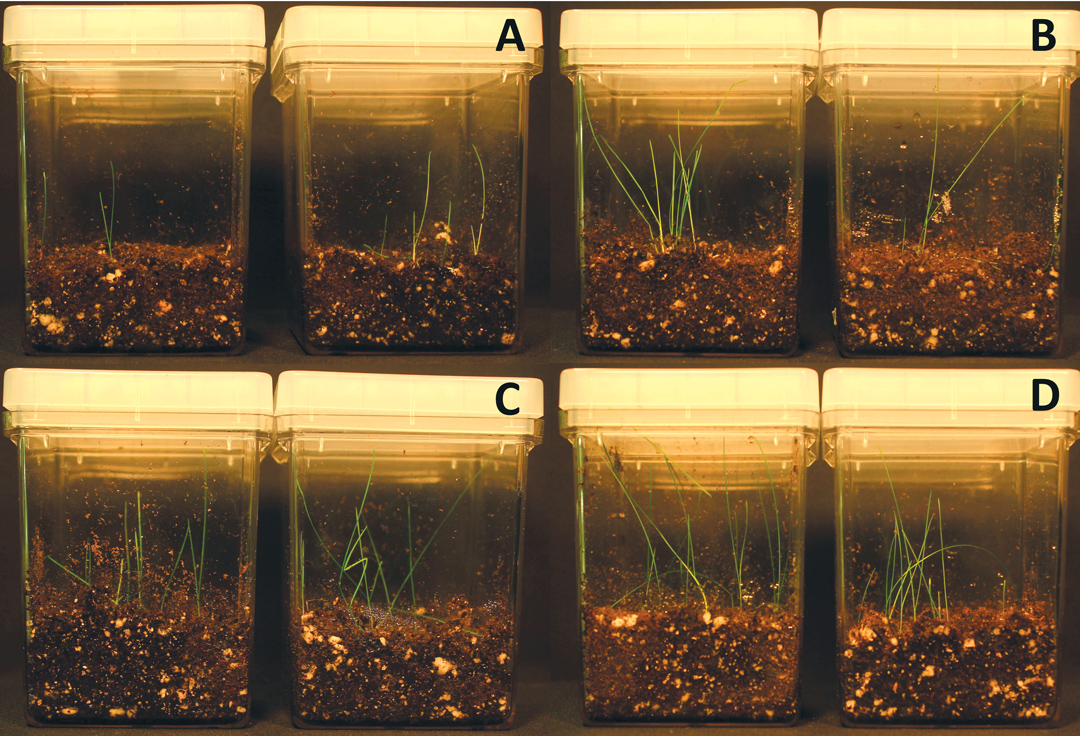
Seeds of Kentucky bluegrass (P. pratensis) were disinfected in 4 percent sodium hypochlorite solution for 30 minutes and rinsed three times with sterile water for 30 seconds. Bacterial isolates Bacillus amyloliquefaciens and Bacillus pumilus from sheeps fescue and two Pantoea agglomerans isolates from tall fescue (F. arundinacea) and teosinte (Zea mays Mexicana) were cultured in Potato Dextrose Brouth. After 24 hours, bacteria were concentrated by centrifugation and then diluted in water to OD600=0.8. Surface disinfected seeds of Kentucky bluegrass were inoculated with bacterial solutions by soaking with agitation for five minutes. Then seeds were placed on sterilized soil with 100mM NaCl. Photos were taken after 20 days germination.
Results and Discussion
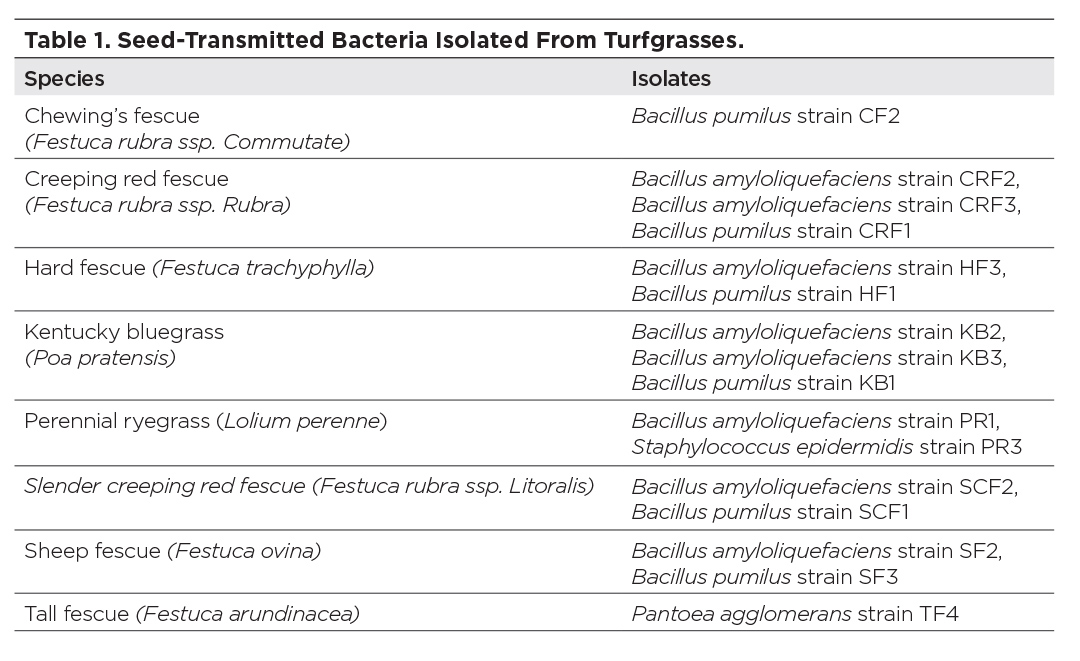
Among grass isolates we identified: Bacillus amyloliquefaciens, Bacillus pumilus, Pantoea agglomerans and Staphylococcus epidermidis. Bacteria most frequently isolated from the grasses were Bacillus amyloliquefaciens and Bacillus pumilus (Table 1).
To evaluate effects of the bacterial endophytes on their hosts, we used four strains (Bacillus amyloliquefaciens strain SF2, Bacillus pumilus strain SF3 and Pantoea agglomerans strain TF and strain T) in Kentucky bluegrass seed germination tests.
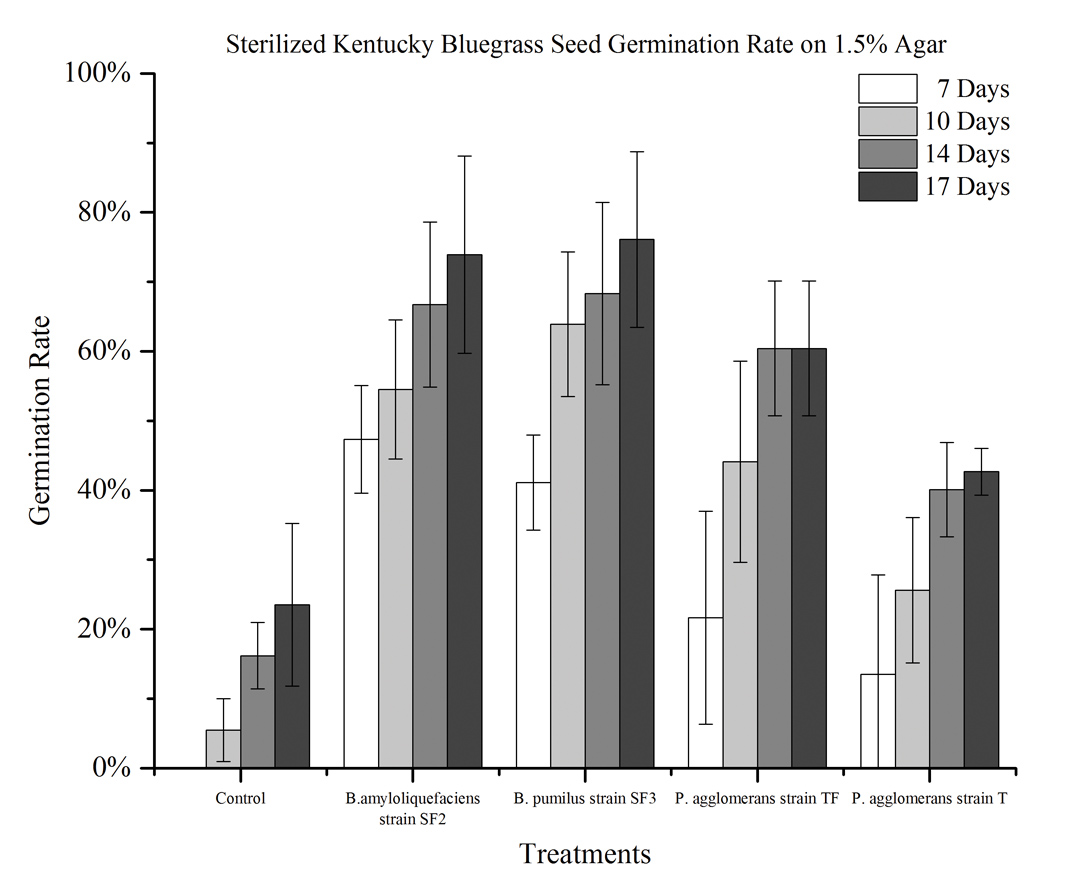
Results from these experiments showed that on 1.5 percent water agar, seeds of Kentucky bluegrass that lacked bacteria had a reduced germination rate compared to seeds where bacteria were added
(Figure 1).
Two Bacillus strains increased the germination rates more than species of Pantoea. Seeds bearing B. pumilus strain SF3 showed the highest germination rate.
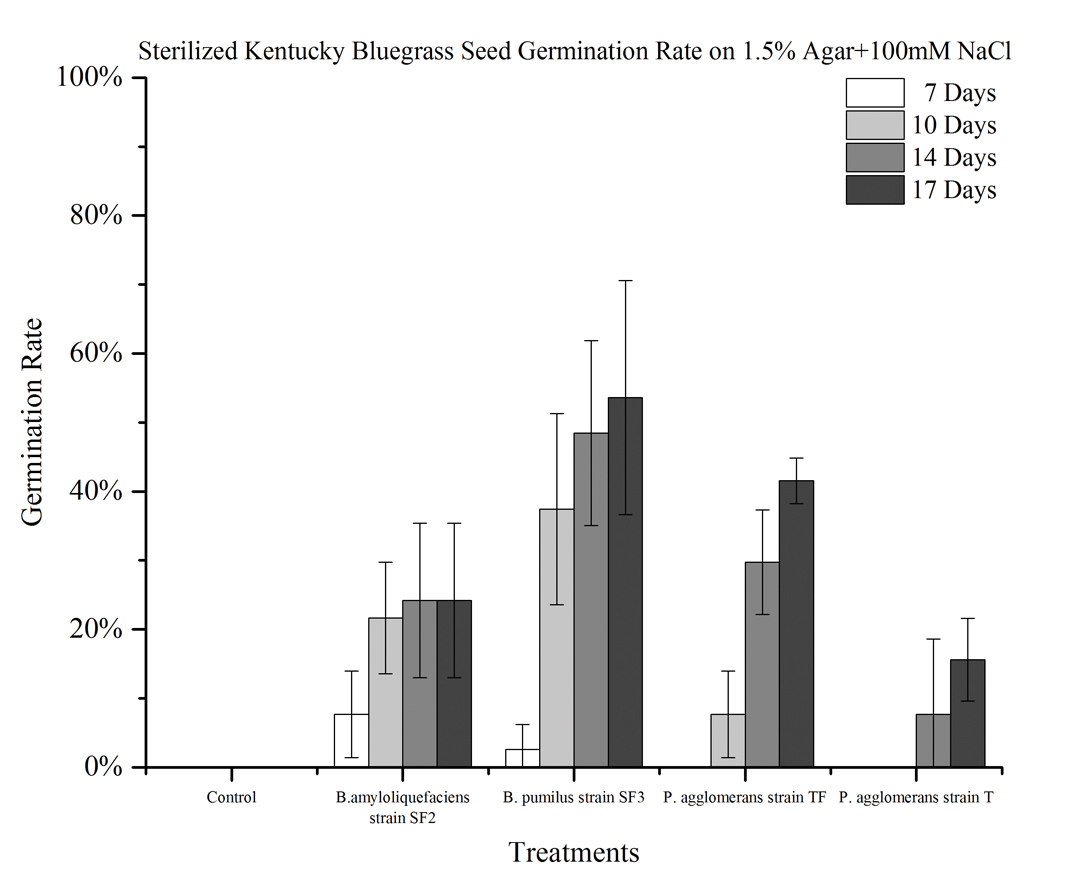
On 1.5 percent agar with 100mM NaCl (salt stress), no seeds germinated in the control. However, Bacillus pumilus strain SF3 promoted seed germination better than the other three bacteria (Figure 2).
In the salinity soil test, grass seeds inoculated with Bacillus amyloliquefaciens strain SF2 and Bacillus pumilus strain SF3 have much better growth than the control (Figure 3).
All these results showed that our bacterial endophytes isolated from turf grasses increase the seed germination rate and promote the seedling growth under high salinity conditions.
Another test on corn (data not shown) demonstrated that the bacterial endophytes from turf Bacillus amyloliquefaciens strain SF2, Bacillus pumilus strain SF3, Pantoea agglomerans strain T and strain TF, promoted root growth of corn.
Based on experiments to date, it seems evident that bacterial endophytes have potential to promote growth and stress tolerance in turf grasses. Our future research will be to:
1) Develop seed treatments for commercial use of Bacillus endophytes in turf; and 2) Evaluate how bacteria alter salt stress tolerance in turf grasses.
References
Adesemoye, A.O., Torbert, H.A., and Kloepper, J.W. 2009. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Micro. Ecol. 58:921-929.
Alshammary, S. F., Qian Y.L. and Wallner, S.J. 2004. “Growth response of four turfgrass species to salinity.” Agricultural Water Management 66: 97-111.
Brem, D. and Leuchtmann, A. 2001. Epichloë grass endophytes increase herbivore resistance in woodland grass Brachypodium sylvaticum. Oecologia, 126: 522–530.
Burelle-Kokalis, N., Vavrina C.S., Reddy M.S. and Kloepper J.W. 2003. Amendment of muskmelon and watermelon transplant media with plant growth-promoting rhizobacteria: effects on seedling quality, disease, and nematode resistance. Hort Technology 13: 476-482.
Carroll, G. 1988. Fungal endophytes in stems and leaves: From latent pathogen to mutualistic symbiont. Ecology 69: 2-9.
Chen, X. H., Koumoutsi, A., Scholz, R., Schneider, K., Vater, J., Sussmuth, R., Piel, J. and Borriss, R. 2009. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol 140(1-2): 27-37.
Chen, X. H., Vater, J., Piel, J., Franke, P., Scholz, R., Schneider, K., Koumoutsi, A., Hitzeroth, G., Grammel, N., Strittmater, A., Gottschalk, G., Sussmuth, R. and Borriss, R. 2006. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J Bacteriol 188(11): 4024-4036.
Chowdhury, S. P., Dietel, K., Randler, M., Schmid, M., Junge, H., Borriss, R., Hartmann, A. and Grosch, R.2013. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS One 8(7): e68818.
Gutiérrez-Mañero, F. J., Ramos-Solano, B., Probanza, A., Mehouachi, J., R. Tadeo, F. and Talon, M. 2001. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol Plant 111: 206-211.
Kuldau, G. and Bacon C. 2008. Clavicipitaceous endophytes: Their ability to enhance resistance of grasses to multiple stresses. Biological Control 46(1): 57-71.
Qian, Y. L. (2003). Salt tolerance should be considered when choosing Kentucky bluegrass varieties. Turfgrass Trends June 2003: 60-64.
White, J. F., Torres, M. S., Sullivan, R. F., Jabbour, R. E., Chen, Q., Tadych, M., Irizarry, I., Bergen, M. S., Havkin-Frenkel, D. and Belanger, F. C. 2014. “Microscopy research and technique: Occurrence of Bacillus amyloliquefaciens as a systemic endophyte of vanilla orchids.” Microsc. Res. Tech.
William A. Meyer, Torres, M.S. and White, J.F. 2012. Biology and applications of fungal endophytes in turfgrasses. Turfgrass: Biology, use and management. John Stier, Brian Horgan and Stacy Bonos, American Society of Agronomy: 713-731.