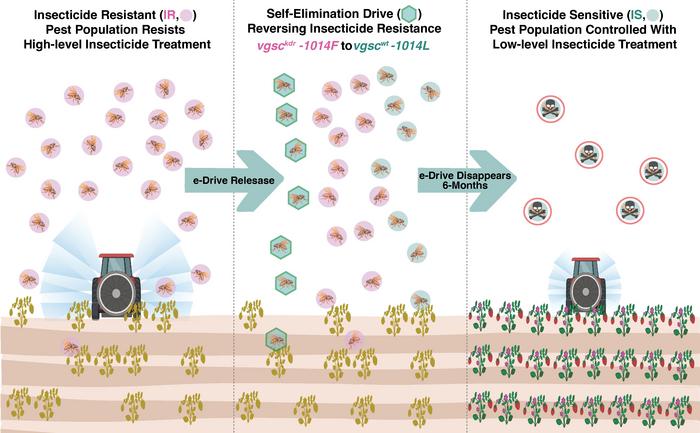
A new genetic system aims to reverse insecticide resistance in pests while minimizing environmental impact and ensuring controlled deployment.
Insecticides have been used for centuries to combat pest damage to essential food crops. Over time, however, insects such as beetles, moths, and flies develop genetic mutations that make these chemicals ineffective.
This escalating resistance forces farmers and pest control specialists to use higher concentrations of toxic compounds more frequently, which poses risks to human health and harms the environment. Many insecticides kill beneficial insects alongside pests, further exacerbating ecological imbalances.
To address these challenges, researchers have developed gene-drive technologies using CRISPR gene editing to replace insecticide-resistant genes with ones susceptible to pesticides. While these tools could dramatically reduce chemical pesticide usage, they have faced concerns about potential uncontrolled spread once released into the environment.
Now, geneticists from the University of California San Diego have introduced a solution to these concerns. In a study published in Nature Communications, Postdoctoral Scholar Ankush Auradkar and Professor Ethan Bier describe a self-eliminating genetic system that reverses insecticide resistance without lasting environmental disruption.
“We have developed an efficient biological approach to reverse insecticide resistance without creating any other perturbation to the environment,” said Bier, a professor in the Department of Cell and Developmental Biology. The system, called a self-eliminating allelic drive, or “e-Drive,” is designed to act temporarily and then disappear, leaving only a population of insects with the corrected gene.
How the e-Drive Works
The researchers developed a genetic “cassette” composed of DNA elements that they inserted into fruit flies for proof of concept. The e-Drive targets a specific gene known as the voltage-gated sodium ion channel (vgsc), which is essential for proper nervous system functioning and often implicated in insecticide resistance.
The cassette uses CRISPR technology to edit the vgsc gene, swapping the resistant variant with its native, insecticide-susceptible form. When flies carrying the cassette mate with others in the population, the e-Drive spreads the corrected gene.
To prevent uncontrolled proliferation, the system imposes a fitness cost on insects carrying the cassette, such as reduced fertility or viability. By inserting the cassette on the X-chromosome, researchers reduced the reproductive success of male carriers, limiting the spread of the cassette over time. Laboratory experiments showed that all offspring were converted to the native gene within 8–10 generations, after which the e-Drive disappeared from the population.
“Because insects carrying the gene cassette are penalized with a severe fitness cost, the element is rapidly eliminated from the population, lasting only as long as it takes to convert 100 percent of the insecticide-resistant forms of the target gene back to wild-type,” said Auradkar.
Potential Applications
The self-eliminating nature of the e-Drive means it can be reintroduced as needed, adapting to different pesticides over time. The team is now working on a similar e-Drive system in mosquitoes to address malaria transmission.
The study also involved collaborators Rodrigo Corder from the University of São Paulo and John Marshall from the Innovative Genomics Institute. Marshall’s mathematical modeling highlighted the system’s efficiency and revealed additional insights into its potential applications.
This groundbreaking technology offers hope for sustainable pest control by reducing chemical pesticide dependence while mitigating ecological and health risks.