It’s crucial the approval process follows legislative timelines, and related issues—such as adventitious presence—are resolved.
BY: Beat Späth
Last year was a very eventful one for GMO authorisations in Europe. The spring saw Member States given license to ban farmers from cultivating EU-approved GMOs, as well as a similar proposal to allow national bans on the use of imported GM crops for food and feed, thus endangering the internal market.
The spring was also the end of an 18-month de facto moratorium, with a record 17 GM crops being approved for import. Later in the year, and after careful examination, Members of the European Parliament rejected the Commission’s proposal on imports as well as a proposal for another moratorium. The year ended with a further two products being approved within the legislative timelines, providing the much-needed hope that the path ahead will be more predictable, stable and science based.
The Ticking Clock
It is clear, in terms of cultivation, the EU has provided Member States with a tool to stop farmers from using approved, innovative products if they, Member States, so decide. For this and other reasons, the demand for, and use of, GM commodities continues to be substantial in the EU. Indeed, every year we import 34 million tonnes of GM soybeans, which equates to the weight of all Europeans put together. European livestock farmers depend heavily on these imports since there is no realistic alternative: the production of soybeans in the EU accounts for 1.7 million tonnes, less than five per cent of EU need.
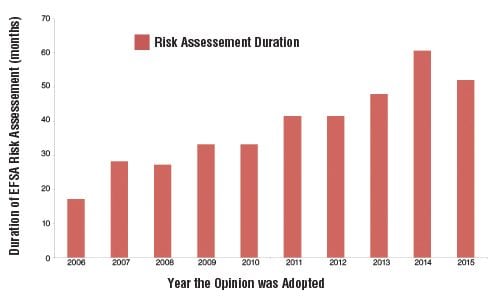
About 70 GMO products are currently authorised for import into the EU, of which 19 were approved in 2015 alone. With such high dependence on imports, it is crucial for the EU to ensure that its approval process follows legislative timelines and is predictable, given the main exporting countries take, on average, less than 2.5 years to obtain a complete GM product approval. Never again should we witness cases where the average approval time in Europe is 6.5 years, as was the case for 17 of the products approved last year.
In order to avoid such cases, it will be important to address the increasing timelines for risk assessment at the European Food Safety Authority level. We are encouraged to see the plans by EFSA to re-establish dialogue through new transparency and engagement projects for 2016. These projects need to resolve issues such as the backlog of products pending at the EFSA level—currently 40 in the GMO area—as well as scientifically unsubstantiated requests to further strengthen risk assessment, and the reduced scientific dialogue between EFSA experts and applicants highlighted in a 2014 letter addressed to EFSA by 11 industry federations across different sectors.
Resolution Needed
In addition to normalising the approval system at the Commission and EFSA levels, it is important that related issues, which have remained unresolved for years and sometimes decades, receive the attention and the resolution they deserve. Indeed, since 2006, Member States have been requesting the Commission come forth with a proposal to address the adventitious presence of GMOs in seeds to redress the fact that there is no internal market for seeds today. Legal thresholds for seeds exist for other admixtures and impurities, including some with hazardous properties, but no threshold is accepted for the adventitious presence of safe GMOs approved elsewhere in the world.
More legal certainty is also necessary for commodity imports where a so-called technical solution for food is urgently sought. The above needs and requests are fully supported by the entire food and feed chain, which has been united in asking for a functioning evidence-based EU policy on GMOs. The most obvious and easy generator of innovation, jobs and growth is to approve safe, new products within legally prescribed and predictable timelines, ensuring consumer safety, while giving industry the consistency it requires to operate.












