Will the new EU Commissions’ “Farm to Fork Strategy” on sustainable food embrace plant breeding innovation?
After the ruling of the European Court of Justice in case C-528/16 on mutagenesis breeding that put all products resulting from any kind of mutagenesis breeding under the GMO definition of the European Directive 2001/18, the seed sector internally discussed on its positioning regarding the way forward.
Euroseeds formally reconfirmed its principal decisions and positions taken in the setting-up of the #EmbracingNature campaign to lead the relevant outreach and advocacy efforts and coordinate communication and advocacy on plant breeding innovation with other industry associations accordingly.

An update of Directive 2001/18 is seen as the only legally possible approach to overcome the challenges that the ECJ ruling put on plants resulting from mutagenesis breeding. This update should be as targeted as possible on the base of excluding those plants from the GMO regulations that also can also result from natural or earlier mutation processes.
Science and Industry Advocate for Updating Europe’s GMO Framework to Current Science
The ruling created strong reactions from scientists who called upon all relevant bodies in the EU and beyond to establish a dedicated international policy forum for further deliberations and collaboration in shaping tomorrow’s GMO governance framework and advancing the public dialogue. Leading scientists representing more than 127 European plant and life sciences research centres and institutes have endorsed a position paper that urgently calls upon European policy makers to safeguard innovation in plant science and agriculture. The scientists are deeply concerned about a recent European Court of Justice ruling around modern genome editing techniques that could lead to a de facto ban of innovative crop breeding.
Young PhD students from Wageningen University even started a European Citizen Initiative “Grow Scientific Progress” to force the EU commission to update the EU GMO legislation. Euroseeds welcomes the citizens’ initiative to ask the European Union to change its GMO legislation in view of targeted mutagenesis breeding. Euroseeds shares the conviction that plant breeding and crop improvement are crucial for all European citizens and key to improve the environmental sustainability and economic competitiveness of the EU’s high-quality agri-food production system. While Euroseeds also shares the objective to change the EU’s GMO Directive to free advanced mutagenesis breeding from the provisions for transgenic crops, we recommend a simpler legal approach to effectively excluding products of old and new mutagenesis breeding from its scope and adapting the respective annex accordingly.
[tweetshare tweet=”Plant breeding and crop improvement are crucial for all European citizens and key to improve the environmental sustainability and economic competitiveness of the EU’s high-quality agri-food production system.” username=”EuropeanSeed”]
Also, business associations from the agri-food value chain expressed their concerns with the ruling and called upon the EU to adapt its legislation to reflect and welcome technical progress and align it with legislation in other parts of the world. Even the scientific advice mechanism (SAM) of the Commission published a quite strong reaction stating that “in view of the Court’s ruling, it becomes evident that new scientific knowledge and recent technical developments have made the GMO Directive no longer fit for purpose.”
Genome edited products might enter the EU market undetected
The outgoing EU Commission started discussions on the consequences of the ruling with EU Member States who are responsible to implement and enforce the GMO Directive. A number of EU Member States addressed concerns with implementing the ruling due to the potential genetic indistinguishability of plants resulting from targeted mutagenesis techniques. The EU Commission therefore requested a report from the JRC and the European Network of GMO Laboratories (ENGL) responsible for the development of detection methods for genetically modified organisms. The main outcome of the report summarizes that based on the current knowledge and technical capabilities, it is unlikely that a detection method for a genome-edited plant product with single nucleotide variations or short insertion/deletions would fulfil the performance requirements for methods of GMO testing. Even in case a DNA alteration is detected, there are currently no procedures established that facilitate an unambiguous conclusion that genome editing has created the alteration versus spontaneous mutation or conventional mutagenesis methods. This means that if a suspicious product with an unknown or non-unique DNA alteration would be detected on the EU market, it would be difficult or even impossible to provide court-proof evidence that the modified sequence originated from genome editing. Therefore, plant products obtained by genome editing may enter the EU-market undetected.
Plant Breeding Innovation can contribute to meeting the new EU Commissions’ goals
With the European Parliament elections in May 2019 and the end of the term of the current EU Commission in late 2019, it was clear that no legislative initiative will be taken until the new Commission is established. This has also been stated repeatedly by the responsible EU Commissioner Andriukaitis and his staff on several occasions.
Euroseeds follows a coordinated outreach and advocacy strategy to build up sufficient political momentum to help initiating a legislative proposal that provides the desired internationally harmonized scope of regulatory oversight.
The new Commission President Ursula von der Leyen included in her strategy for Europe to strive for more by being the first climate-neutral continent. In this context she wants to implement a Biodiversity Strategy for 2030, a “Farm to Fork Strategy” on sustainable food as well as a new Circular Economy Action Plan. Plant breeding innovation can contribute to all these goals and Euroseeds will definitely address the topic in this context in view of our communication activities as well as our advocacy efforts.
[tweetshare tweet=”Plant breeding innovation can contribute to the goal of “Farm to Fork Strategy” on sustainable food.” username=”EuropeanSeed”]
Finland is, as current Council Presidency, following up on the Dutch proposal that was presented in the AgriFish Council in May 2019 to call upon the incoming new Commission to include addressing the adequacy of the European legislative framework for GMOs – and as appropriate other related legal and policy instruments – in its program of work. One major driver for this proposal was the above mentioned JRC/ENGL report questioning the ability of member states to enforce the current regulation with regard to products that cannot be distinguished as described above. The Dutch proposal was supported by a number of member states. After the Council meeting EU Farm Commissioner Phil Hogan said his colleague Vytenis Andriukaitis (responsible for Health & Food Safety) continues to get advice on the legal advice on mutagenesis techniques & was asking national capitals for “certain data” to help the Commission to come up with a “robust response.”
The Finnish Council presidency recently put forward a follow up-proposal to ask the Commission to conduct a study addressing the legal situation of new plant breeding techniques taking into account the existing legal framework for GMOs provided by Directive 2001/18/EC and the Court of Justice’s judgment in Case C-528/16. The study should be accompanied by a proposal, if appropriate in view of the outcomes of the study, or otherwise to inform the Council on other measures required as a follow-up to the study.
Can Europe afford to be left behind?
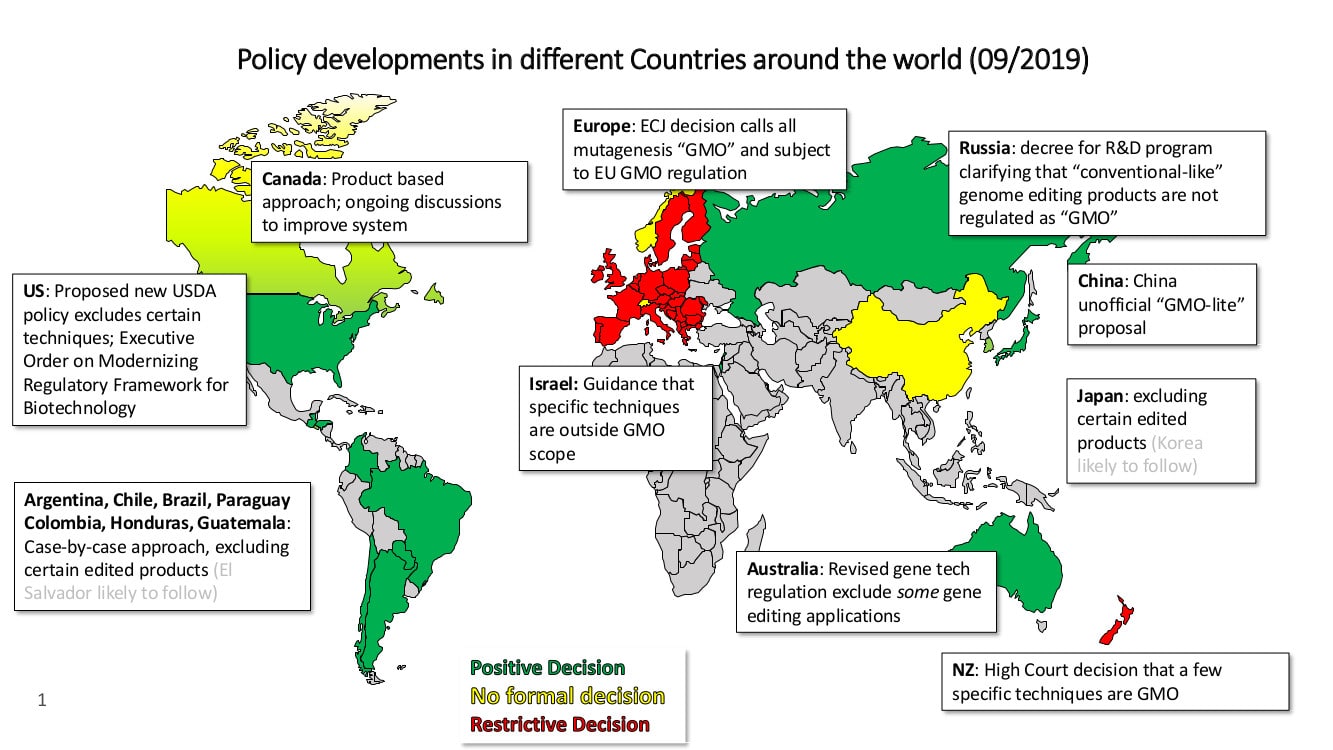
Currently, there is a trend towards a global harmonization of regulatory oversight when it comes to the products of new breeding methods including genome editing. Most countries which already implemented or discussed new or updated policies follow the principle approach that products including a novel combination of genetic material as laid out in the LMO-definition of the Cartagena Protocol will be in the scope of their biotech regulations. This includes several South American countries like Argentina, Colombia, Brazil, Chile, Honduras, Guatemala and Japan. Also, the US, Australia and Russia exclude certain products of genome editing from their biotech regulations (for more information: see Jorasch, 2019 a[i]).
Differing regulatory requirements will limit the capacity of the industry to innovate. It will also reduce the availability of genetic resources for breeding and have a negative effect on research collaborations as well as hinder the movement of seed globally. In addition, commodity trade disruption will occur, and agricultural development and food security will be impeded. Enforcement issues, like the ones mentioned above are likely to increase because seeds and commodities developed with the aid of some of the latest plant breeding methods may be indistinguishable from those derived from traditional plant breeding methods or naturally occurring genetic variation.
A harmonized scope of regulatory oversight would accommodate current scientific progress and help addressing significant global challenges like climate change and food security in a timely manner. In order to achieve the new Commissions’ goals Europe should welcome innovation and not fall behind the rest of the world in terms of adopting innovation and the broad societal benefits brings (Jorasch 2019 b[ii]).
[i]Jorasch, P. (2019a) in press: Will the EU stay out of step with science and the rest of the world on plant breeding innovation? DOI: 10.1007/s00299-019-02482-2
[ii]Jorasch, P. (2919b) The global need for plant breeding innovation; Transgenic Research Vol. 28, (2), pp 81–86 https://doi.org/10.1007/s11248-019-00138-1