Research may lead to more sustainable food supply.
Scientists have unveiled the gene that enables marine algae to synthesize a unique type of chlorophyll, and they have successfully transferred the gene into a land plant, setting the stage for improved crop yields on reduced-land areas.
This discovery, described in a recent news release from the University of California-Riverside, resolves a long-standing mystery among scientists regarding the molecular pathways that allow algae to produce this chlorophyll and survive.
“Marine algae produce half of all the oxygen we breathe, even more than plants on land. And they feed huge food webs, fish that get eaten by mammals and humans,” UC Riverside assistant professor of bioengineering and lead study author Tingting Xiang said in the release. “Despite their global significance, we did not understand the genetic basis for the algae’s survival, until now.”
The study, published in Current Biology, also showcases another groundbreaking achievement: demonstrating that a land plant can produce marine chlorophyll. Tobacco plants were used in this experiment, but theoretically, any land plant could incorporate the marine algae gene, enabling them to absorb a broader spectrum of light and achieve enhanced growth.
Chlorophyll is the pigment that facilitates photosynthesis, the process of converting light into chemical energy. Plants produce chlorophyll a and b, while most marine algae and kelp produce chlorophyll c, which allows them to absorb the blue-green light that reaches the ocean depths.
“Chlorophylls b and c absorb light at different wavelengths,” said Xiang. “The ocean absorbs red light, which is why it looks blue. Chlorophyll c evolved to capture the blue-green light that penetrates deeper into the water.”
This research could also have implications for algae biofuel production. Some algae species produce chlorophylls a or b, like land plants, instead of c. Equipping these algae with the gene to make chlorophyll c could enhance their ability to utilize more light and to increase their growth, creating more feedstock for biofuels.
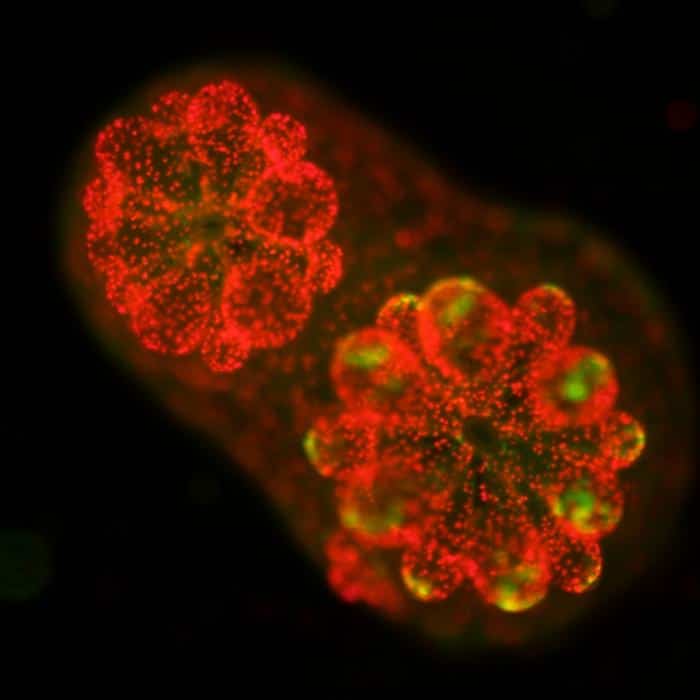
The researchers initially aimed to gain insight into an algae species that lives in coral. These algae produce sugars and share them with their coral hosts. “Each coral colony has thousands of polyps, and their brown color is from the algae. Whenever you see coral bleaching, it’s due to the loss of the algae,” Xiang said.
In studying mutant algae that were more yellow than brown and unable to photosynthesize, the researchers found that these mutants could still survive and grow in coral, as the coral provides the algae with the nutrients needed for growth.
By using next-generation DNA sequencing and extensive data analysis, the researchers were able to identify the gene responsible for chlorophyll c production using these mutants. “Discovering the chlorophyll c gene was not the initial goal of our work. We made the mutants for another reason, but I guess we were just lucky,” Xiang said.
With newfound knowledge of the genetic basis for producing chlorophyll c, the researchers hope their work could help address the global issue of coral bleaching and support land-based strategies to adapt to climate change.
“The identification of the biosynthetic pathway for chlorophyll c is more than a scientific curiosity; it’s a potential game-changer for sustainable energy and food security,” said Robert Jinkerson, UCR chemical engineering professor and study co-author.
“By unlocking the secrets of this key pigment, we’re not only gaining insights into the lifeblood of marine ecosystems but also pioneering a path towards developing more robust crops and efficient biofuels,” Jinkerson said.